american academy of pediatrics covid vaccine under 12
The American Academy of Pediatrics has strongly recommended since May that 12- to 17-year-olds that are eligible to get the vaccine be vaccinated Shrifin says. COVID-19 vaccines have been available for children ages 5-11 since October 29 2021.

More Children Are Catching Covid 19 But It S Unclear If They Re Getting Sicker Coronavirus Updates Npr
The viral vector vaccine in the United States is given in one dose to people 18 years and older.
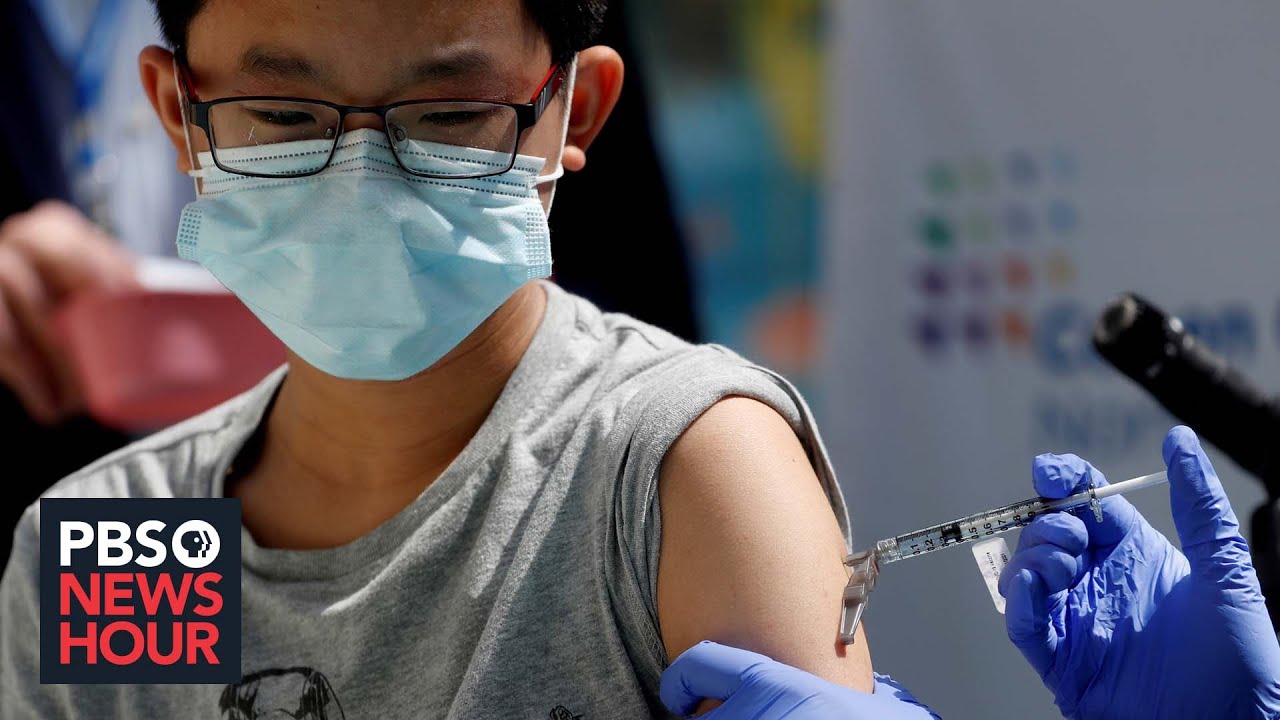
. Vaccines for children age 6 months and up may be authorized next. The CDC reported more than 400 kids under 5 have died of COVID-19 many in the past few months. Military would be required to have the COVID-19 vaccine beginning Sept.
With the delta variant raging almost five times as many. With the delta variant raging almost. Regardless of the reason low efficacy against infection is one more reason why a parent might resist vaccinating their children in the first.
Just twenty-six percent of 511-year-olds. Ad Maximize protection get all recommended vaccine doses and boosters as soon as possible. The vaccine will continue.
Clinical trials are underway for children age 6 months to under 5 years old. COVID-19 vaccine effective for kids under 5 According to the American Academy of Pediatrics nearly 128 million kids have tested positive for COVID-19 since the onset of the pandemic. Preparing Children and Teens for Vaccination.
Adults have received at least one vaccine dose and just about everything has opened up the American Academy of Pediatrics reported over 250000 new. FDA authorizes COVID-19 vaccine for adolescents ages 12-15. 10 hours agoCOVID vaccines would be required for military under new plan Members of the US.
ITASCA IL -- Today the Food and Drug Administration FDA finalized the full approval of the Pfizer-BioNTech COVID-19 vaccine for ages 16 and older. Novavax Inc said on Tuesday its COVID-19 vaccine has got emergency-use authorization from the Drugs Controller General of India for children aged 12 to 17 years. Currently a booster shot is not recommended for children younger than 12 years old.
BIDDEFORD Maine The American Academy of Pediatrics is urging the Food and Drug Administration to approve a COVID-19 vaccine for children under the age of 12 as soon as possible. Murphy Senate Colleagues Urge FDA to Quickly Review COVID-19 Vaccines for Children Under 12 After recent data shows exponential increase in COVID-19 cases in children senators urge FDA to work as quickly and aggressively as possible to authorize safe and effective COVID-19 vaccines for children under the age of 12 September 29 2021. Data released Wednesday by Moderna showed two-doses of its 25 microgram vaccine is safe and.
A COVID vaccine is available for children age 5 and up and boosters are now authorized for those 12 years and older. Have been shown overall to be safe and highly effective. NPR As the US.
The dose may be different for younger ages. As of March 16 2022 the Centers for Disease Control and Prevention CDC reports that just one-third 33 of children in this age group have received their first vaccine dose with vaccination rates varying widely by state. NPRs Ailsa Chang speaks with American Academy of Pediatrics President Lee Savio Beers about the mounting pressure to consider emergency use authorization of COVID-19 vaccines for children under.
Research shows these new vaccines are remarkably effective and safe. The American Academy of Pediatrics wrote a letter Thursday to the US. The AAP recommends against giving the vaccine to children under 12 years until authorized by the FDA The FDAs licensure of the vaccine which will be called Comirnaty applies to ages 16 years and older the group for whom emergency use authorization EUA was granted in December 2020.
Until COVID-19 vaccines are made available to younger students experts recommend that they continue to take other protective measures. As the US. The authorization is a global.
While questions may now arise about the administration of the vaccine off-label for children aged 11. While the Covid vaccines in use in the US. Continues inoculating adults and adolescents questions remain about vaccinating the 48 million kids under the age of 12.
Continues inoculating adults and adolescents questions remain about vaccinating the 48 million kids under the age of 12. A single booster dose of the Pfizer vaccine is authorized for kids 12 through 17 years old at least 5 months after the second dose of vaccine. At a time when more than 75 percent of US.
American Academy of Pediatrics Cautions Against Off-Label Use of COVID-19 Vaccines in Children Under 12. Teens ages 12 to 17 years old should receive the Pfizer-BioNTech COVID-19 booster shot at least 5 months after completing their Pfizer-BioNTech COVID-19 primary series. State reports show that about 128 million kids have tested positive for COVID-19 since the start of the pandemic according to the American Academy of Pediatrics.
Children accounted for 15 of new Covid cases reported last week American Academy of Pediatrics data analysis shows A 13-year-old shows his bandage after receiving a dose of the Covid vaccine in. AAP CDC recommend COVID-19 vaccine for ages 12 and older May 12 2021 In addition to approving the Pfizer-BioNTech vaccine for adolescents the CDC updated its clinical guidance to allow coadministration of vaccines. 15 under a plan announced by the Pentagon.
The best way to slow the emergence of COVID variants is to get vaccinated. Only the Pfizer-BioNTech Covid vaccine has been authorized for adolescents and teens ages 12-17. Food and Drug Administration highlighting that the Delta variant has created a new and pressing risk to children under age.

Kids Covid Vaccine Twitter Chat Twitter
Covid Vaccines For Kids Under 12 Expected Midwinter Fda Official Says
What Parents Should Know About Covid 19 Vaccines For Kids Under 12

Fda Again Warns Parents Not To Get Children Under 12 Vaccinated Yet The New York Times

American Academy Of Pediatrics Urges Fda To Approve Covid Vaccines For Children Under 12 Youtube
Aap Advises Children And Teens Age 12 And Up Get The Covid 19 Vaccine Healthychildren Org

Pediatricians Want Kids To Be Part Of Covid Vaccine Trials Kaiser Health News

What To Know As Covid Vaccines For Kids Under 12 Face Final Hurdles Nbc Chicago
Pediatricians Besieged By Parents Seeking Coronavirus Shots For Kids Under 12 The Washington Post
Faq About Pfizer S Covid Vaccine And Kids Shots Health News Npr
What Pediatricians Want Parents To Know About The Covid Vaccine For Kids
Pfizer Biontech Ask Fda To Authorize Coronavirus Vaccine For Children 5 To 11 The Washington Post
Nearly 1 Million Kids Under Age 12 Have Had The Covid Vaccine Wh Says

Kids And Covid Vaccines The Fda Timeline Booster Recommendations And More Info Cnet
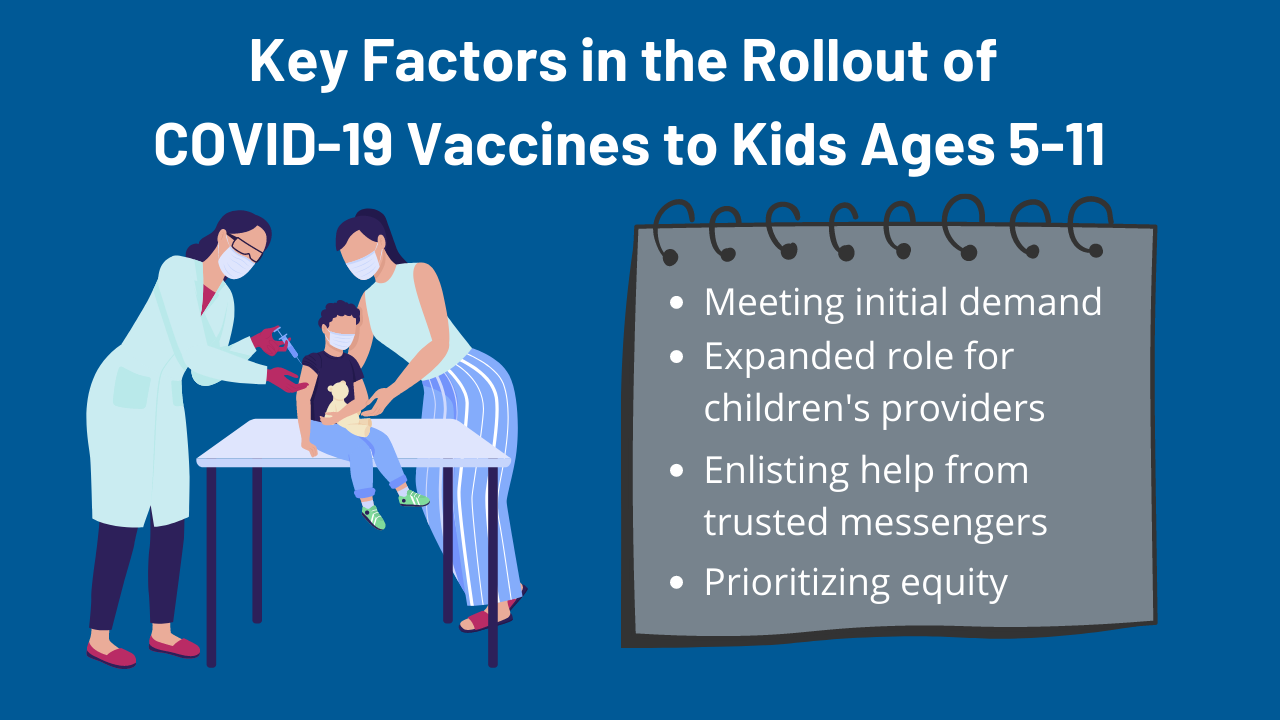
Vaccinating Children Ages 5 11 Policy Considerations For Covid 19 Vaccine Rollout Kff

Faq Covid 19 Vaccine For Kids Under 12 Lurie Children S

What To Know About Children 5 11 Getting The Covid Vaccine The Washington Post

Fact Check How Useful Are Coronavirus Vaccines For Children And Adolescents Coronavirus And Covid 19 Latest News About Covid 19 Dw 06 08 2021

As Omicron Surges Effort To Vaccinate Young Children Stalls Kaiser Health News